Sterilization depends on contact of the sterilizing agent with all surfaces of the item being treated. The type of agent used depends upon the nature of the item to be sterilized and the amount of time required to kill the unwanted spores is critical.
Steam Sterilization
No living thing can survive direct exposure to saturated steam at 250 F (120 C) longer than 15 minutes. Heat destroys microorganisms, but this process is hastened by the addition of moisture, in the form of steam. Pressure is necessary to increase the temperature of the steam for sterilization.
Steam must penetrate every fiber and reach every surface of the items to be sterilized. When steam enters the sterilizer chamber under pressure, it condenses upon contact with cold items. This liberates heat, simultaneously heating and wetting all of the items in the load and thus providing the two requisites: moisture and heat.

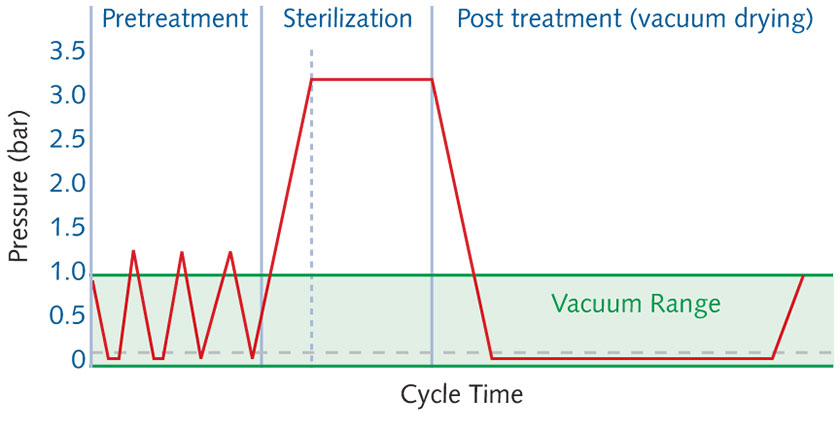
The steps of steam sterilization are:
- Pretreatment – all air is evacuated, using a vacuum pump, and replaced with steam
- Sterilization – the goods are heated by introducing steam and having it condense on all surfaces, heating and humidifying the spores
- Post Treatment – the process is finished during this third phase, during which the steam is evacuated by applying deep vacuum. This is often called the drying phase because during this process the condensate boils off and the vacuum pump takes it away.
Hydrogen Peroxide Plasma Sterilization
In this method, hydrogen peroxide is activated to create a reactive plasma or vapor. The cloud of plasma created consists of ions, electrons, and neutral atomic particles that produce a visible glow. The plasma and vapor phases of hydrogen peroxide are highly effective at killing microorganisms, even at low concentrations and temperature.

The steps involved are:
- sealing the articles to be sterilized into the vacuum chamber
- evacuating the vacuum chamber
- creating a gas discharge plasma
- injecting reactive agent into the vacuum chamber
- diffusing reactive agent
- partially evacuating the vacuum chamber
- generating a reactive agent plasma
- removing gas or vapor from the articles in the chamber
- venting vent gas into the vacuum chamber
- evacuating the vacuum chamber
- venting vent gas into the vacuum chamber
- removing the sterilized articles from the chamber
Ethylene Oxide Sterilization
Ethylene oxide (EO) is used to sterilize substances that would be damaged by high temperature techniques such as pasteurization or autoclaving. Used as a gas, EO gas must have direct contact with microorganisms on or in items to be sterilized. Because EO is highly flammable and explosive in air, it must be used in an explosion-proof sterilizing chamber in a controlled environment. Also, it takes longer than steam sterilization, typically 16-18 hrs. for a complete cycle.
EO gas sterilization is dependent upon four parameters: EO gas concentration, temperature, humidity, and exposure time. Each parameter may be varied. The sterilization chamber has most of its oxygen removed (to prevent an explosion) and is then flooded with a mixture of ethylene oxide and other gases, which are later aerated.

The basic steps are:
- deep vacuum
- humidification
- gas admission
- sterilization
- pulse post vacuum
- aeration
- equalization
Formaldehyde Gas Sterilization
Formaldehyde is used as a fumigant in gaseous form. Formaldehyde sterilization is complex and less efficacious than other methods of sterilization. It should only be used if steam under pressure will damage the item to be sterilized and ethylene oxide and glutaraldehyde are not available. Its use for sterilization has been almost abandoned in the United States, Canada, and Australia, but it is still used in Europe and Asia.

Ozone Gas Sterilization
Ozone, a form of oxygen, sterilizes by oxidation, a process that destroys organic and inorganic matter. Ozone is an unstable gas, but can be easily generated from oxygen. A 6 to 12 percent concentration of ozone continuously flows through the chamber. Penetration of ozone may be controlled by vacuum in the chamber, or enhanced by adding humidity. At completion of exposure time, oxygen is allowed to flow through the chamber to purge the ozone. Cycle time may be up to 60 minutes depending on the size of the chamber or the load.